Microfluidizer® High Shear Fluid Processors for Pharmaceutical Nanoemulsion Formulations
Microfluidizer® High Shear Fluid Processors produce stable nanoemulsions of two or more immiscible liquids with extremely small droplet size, using the highest shear forces. Force is applied uniformly so that every millilitre undergoes the same constant, controlled shear rates for tight particle size distribution.
With a wide range of Microfluidizer® processors available that are simple to use and clean, and conform to cGMP requirements, our nanoemulsion formulation solutions from Microfluidics™ are trusted for research, development and production in different pharmaceutical applications across the globe.
Key advantages:
- Stable dispersions with a long shelf-life
- Well-defined, narrow Particle (droplet) Size Distribution (PSD)
- Formulations compatible with effective sterile filtration
- Optimised bio-availability through particle size control
- Fewer passes required, saving time and money
- API material integrity maintained with efficient temperature control
- Reliable Clean-in-Place and GMP compliance available from lab-scale to production
- Low maintenance & minimal down-time


Achieve Tight Particle Size Distribution with Microfluidizer® High Shear Processors
Comparison between the Microfluidizer® and standard high pressure homogeniser in terms of particle size and PSD
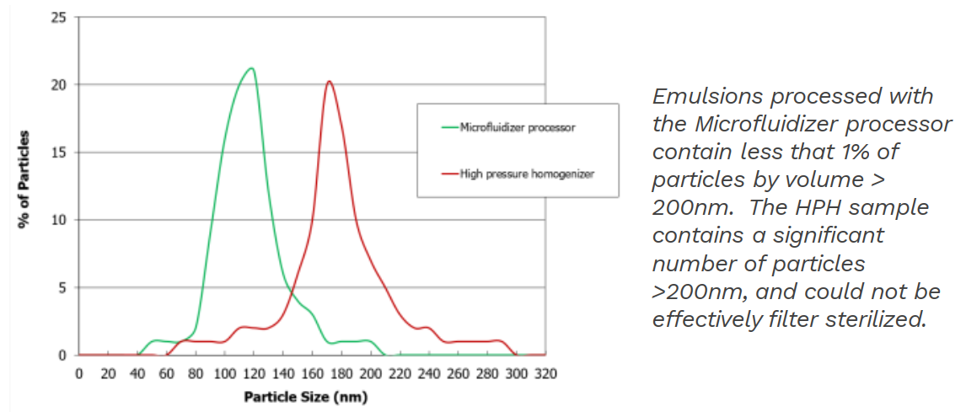
Filterability: The route of administration requires parenteral nanoemulsions to be sterilized. An effective and relatively simple sterilization method is filtration through 220nm sterilizing-grade filters, also known as terminal sterilization. This is made possible using the Microfluidizer® high shear homogenizer which produces particle with the less than 1% by volume being greater than 200 nm.
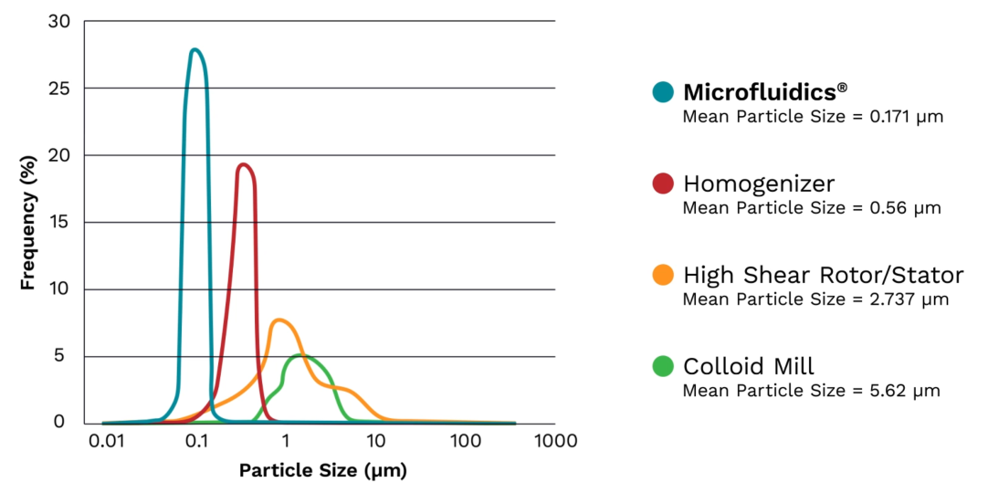
Comparison of Microfluidizer® PSD with other technologies
Case Study
Comparison of the unprocessed Propofol emulsion before processing through the Microfluidizer® at just 20,000 psi and after processing.
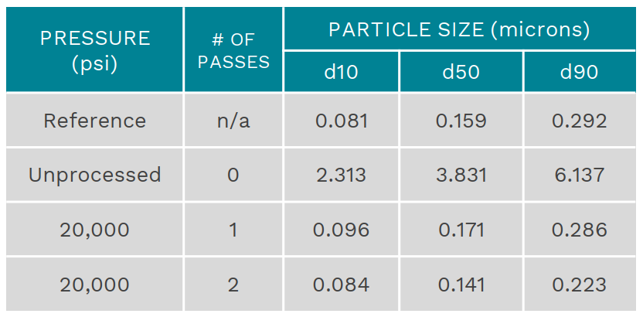
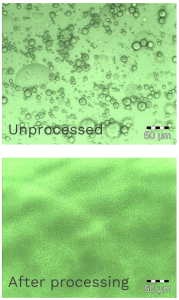
Emulsion before and after being processed through a Microfluidizer® high shear processor
- As with all other parenteral/injectable drug products, Propofol emulsion must be sterilized to destroy or remove any potential microbial contaminants.
- Propofol is a stable drug at high temperatures, which means the emulsion can be autoclaved to achieve sterilization.
- With Microfluidizer® technology, the d90 particle size indicates that the majority of droplets inside the processed emulsion are small and similar to the mean size of the sterile filters (220 nm), therefore this emulsion may be sterile filtered instead of having to be autoclaved.
Summary of the advantages of the Microfluidizer® High Shear Processing System
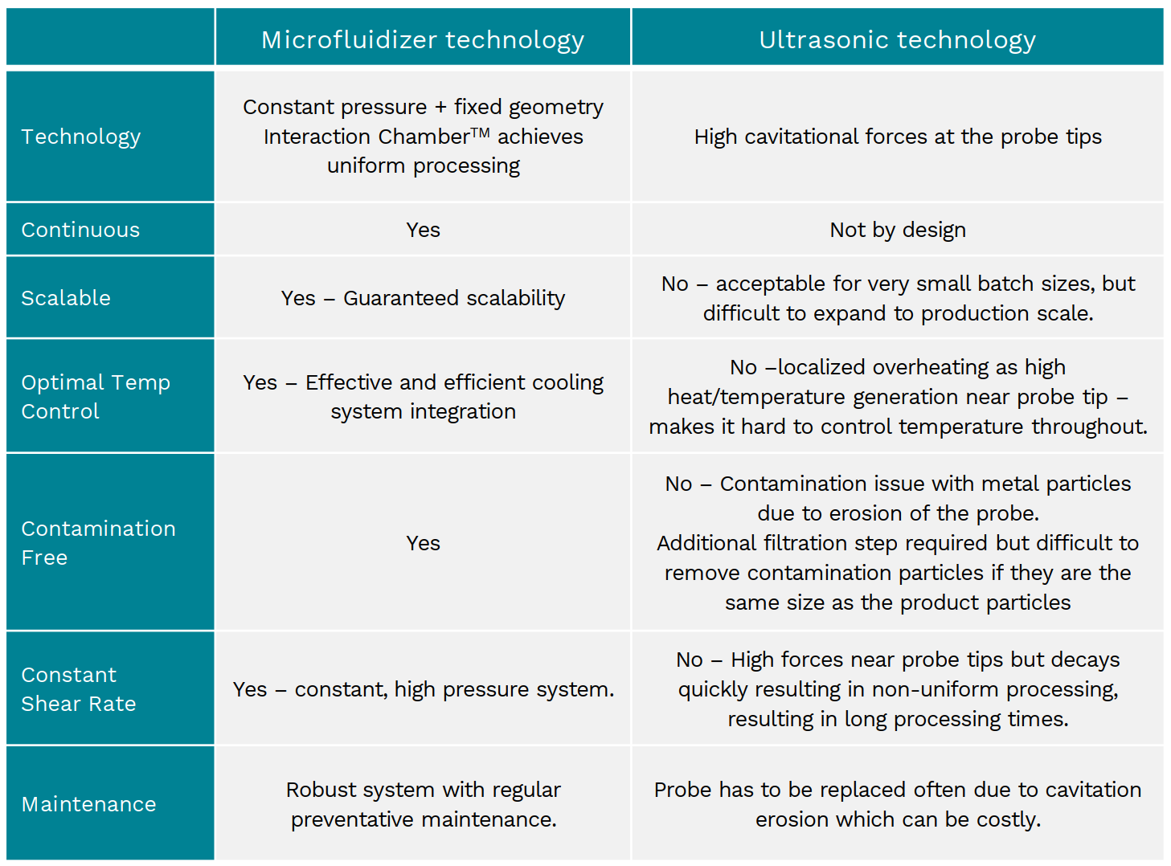
Microfluidizer® versus High Pressure Homogenizer
In a study of a nanoemulsion intended for sterile filtration, the Microfluidizer® significantly out-performed the HPH:
Power consumption – The Microfluidizer® processor consumed 7.5 times less power than the HPH.
Efficiency – Nanoemulsions processed through the Microfluidizer® had particle sizes that were 18-55% smaller than the HPH when both systems were run at the same energy input.
Uniformity – nanoemulsions up to 90% less polydisperse than the HPH run at the same energy input.
Repeatability – the particle size standard deviations of the nanoemulsions were much lower when produced by the Microfluidizer® processor (0.1-2.6) compared to the HPH (3.8-14.8).
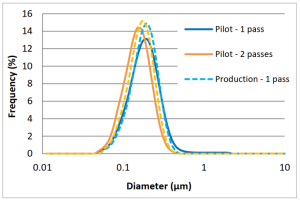
Scalability – From Pilot to Production
The chart here shows how the results from the pilot scale M110EH model were replicated in the M7250-30 production model. Particle sizes after both 1 and 2 passes obtained on the production machine were very close to that obtained on the pilot machine. The chart also shows how tight the particle size distribution (PSD) is that can be achieved.

cGMP Manufacturing
Microfluidics have more than 15 years’ experience with cGMP requirements for pharmaceutical applications and are trusted globally for their in-depth professional knowledge, expert support, and high-quality biopharmaceutical manufacturing equipment, for the most effective submicron particle size reduction and optimal yield for all your biotech and pharmaceutical productions.